Summary
Evidence suggests that low indoor humidity is the main reason that cold and flu viruses peak during the winter. Because of low humidity, these viruses remain airborne up to 27 times longer in the winter. The SARS-CoV-2 virus has properties similar to other coronaviruses that cause the common cold and to flu viruses. This suggests that low humidity in indoor situations could contribute to SARS-CoV-2 transmission. If so, putting people in lockdown could be counterproductive unless we raise the humidity of our home, shopping, and workplace environments to dampen airborne transmission. Calculations show that increasing humidity could reduce R0 significantly.
 t may surprise you to learn that, while everyone has a theory, nobody really
knows why colds and influenza hit us in the wintertime. We were always taught
that it's because people are cooped up indoors, or maybe heat and sunlight
kill the virus. But research has now shown that it's not the heat,
it's the humidity.
t may surprise you to learn that, while everyone has a theory, nobody really
knows why colds and influenza hit us in the wintertime. We were always taught
that it's because people are cooped up indoors, or maybe heat and sunlight
kill the virus. But research has now shown that it's not the heat,
it's the humidity.
It's important to know. If being cooped up causes influenza to spread, then being in lockdown could actually be the worst possible strategy. Maybe we ought to go out to the mountains and practice skiing instead of staying at home updating our blogs while pretending to work. (Almost all research on other diseases has screeched to a halt. It's not so easy doing experiments over a VPN.)
This is not to say that the virus can't spread in warm, humid climates. But the scientific evidence is clear that when humidity is low, airborne transmission of certain viruses indoors is more efficient, and the viruses remain viable longer. Whether this is also true for SARS-CoV-2 remains to be seen. (If someone claims that the facts below are fake news, we need to hear from them in terms of molecular physics why they think the researchers in PNAS and Annual Review of Virology are mistaken.)
There are other theories of course, such as absence of vitamin D and changes in intrinsic barriers in the host such as mucus. Science magazine has a great graphic showing that not all contagious diseases are highest in the winter. In the times when smallpox, chickenpox, and rubella were pandemic they were highest in May, while hepatitis A and polio were highest in August, measles in April,[1] and human coronaviruses and respiratory syncytial virus (RSV) in winter.[9]
The biggest factor in determining seasonality seems to be how the environment affects the physical properties of the virus particle. Not counting mosquito-borne viruses, the primary driver, according to Shaman and Lipsitch,[2] is not heat or ultraviolet light, but humidity. The popular idea that viruses are inactivated by heat, which led a politician from—where else—Florida to try to cure himself by blasting hot air from a hair dryer up his nose, is not supported by more rigorous experimentation (although, to be fair, there are few randomized clinical trials on it, and perhaps we ought to start one).
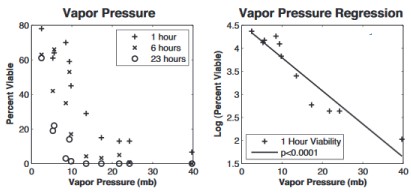
Influenza virus survival vs humidity as measured by water vapor pressure. From Shaman & Kohn, PNAS 2009. [3] Scale is loge; 4.5 = 90% survival, 1.5 = 4.5% survival
Scientists warn that blasting a hair dryer up your nose should be left to professionals. Their research shows that low absolute humidity, which occurs indoors during the winter, is highly correlated with influenza virus survival[3,4] (see graph at right). Correlations with other factors, such as temperature and relative humidity, are much weaker, suggesting that absolute humidity, not temperature, is the main driver.
At the end of the paper the authors say[3] global warming will increase absolute humidity and reduce influenza virus survival and transmission rates. So global warming would be highly beneficial as far as the flu is concerned.
Once again, these are not my theories and they apply only to airborne transmission in a closed space. How long influenza viruses remain viable on surfaces or in droplets is outside the scope of this article.
SARS-CoV-2 vs Influenza and cold viruses
We don't know for sure whether the same is true of SARS-CoV-2 (the Wuhan coronavirus), but previous SARS outbreaks always occurred in cold winter seasons after a severe drought, suggesting that low humidity and cold temperatures are conducive to survival and transmission of both viruses.[8] MERS-CoV was also first detected in Jeddah, Saudi Arabia during a hot period when no rain had fallen for a month.[8] This is strong circumstantial evidence that humidity but not heat plays a critical role.
Because most people spend 90% of their time indoors, the old idea that people are more closely packed together in winter is no longer true. However, in winter, low outdoor absolute humidity translates to low indoor relative humidity. It's relevant that rhinovirus infections are most common in September and October, while HCoV infections are most common in February, even though they both cause the common cold[9]—suggesting that it's the physical properties of the virus that make the difference.
Amazingly enough, researchers in Taiwan[10] modeling influenza air transmission published an equation that lets us estimate R0:
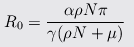
where μ is the virus elimination rate, ρ is the chance of picking up the virus in persons−1day−1, N is the total population in the infected area, α=pathogens/person/day, γ=recovery rate/day, and π=infectivity.
Taking values of N=1000, ρN=0.0877 and μ=8.64 from their earlier paper[11], we can estimate that increasing μ by a factor of 27 would reduce R0 by a factor of 21.4.
This needs to be verified empirically, and it assumes all transmission is through aerosols and not from touching fomite (contaminated towels and surfaces). If people are touching each other or contaminated objects, all bets are off.
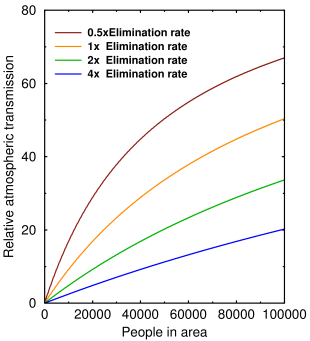
The graph above shows how airborne transmission changes with the virus elimination rate μ and the number of people packed together. Virus elimination rate is determined by the humidity and the exposure rate is determined by the population density and the intrinsic transmissibility of the virus.
If there's only one person, no transmission can occur. As more people pack in, the three curves will eventually converge and the elimination rate doesn't matter. At realistic population densities (≈1000–10000), even doubling the elimination rate by increasing the humidity can have a big effect.
It also tells us, as everybody knows, that population density plays an important role in airborne transmission. But if people want to ‘flatten the curve’, this is the curve they should be flattening.
Exactly why low humidity protects these viruses and not others is unknown. Some researchers suggested that humidity affects the pH or the lipids on the virus envelope.[5] But a recent study found that influenza viruses live longer and travel farther when the air is dry because their equilibrium size is smaller: at 100% relative humidity, droplets took 8 minutes to settle on a surface 1.5 meters away, while at <64% relative humidity they took 216 minutes and had six times higher deposition efficiency in the alveolar region.[5] So viruses float around 27 times longer when humidity is low, which—if true—means the air in your home or office is 27 times as dangerous in the winter.
Unfortunately, there's a catch: invasive aspergillosis is known to complicate severe influenza.[6] Bacterial super-infection of influenza patients is also a serious problem, which is why antibiotics are often given despite their minimal effect on the virus. The ideal humidity for preventing aerosol virus transmission is between 40% and 60%.[9] So if you turn the humidity so high that you get mold growing on the walls and water dripping off your windows, you might want to back off a bit.
But the implications are clear: putting people in lockdown could be dangerously counterproductive unless we raise the humidity of our environment to dampen airborne transmission. Stated another way, it would reduce airborne transmission, and therefore R0, leaving direct contact of droplet spray and fomite (indirect, from towels and surfaces) as the only means of virus transmission.
A few stores do control their humidity, but I've been in grocery stores where you'd get a static shock in winter every time you touch the display case. Changing this wherever people congregate would be simple and inexpensive.
El Niño
The seasonal connection is so strong that even El Niño correlates with influenza patterns.[7] Here's how that works: wild birds are the primary reservoir of influenza A viruses, and El Niño changes their numbers and flying patterns. The inventors of this theory say
Migratory birds, with their long travel distances and many stopovers, are thought to be particularly critical for the mixing and reassortment of influenza virus genomes.
So, the theory goes, by changing bird population density and flight and stopover patterns, El Niño can bring divergent influenza subtypes together and favor the creation of new pandemic strains. Shaman and Lipsitch[7] found that all four major influenza pandemics (1918, 1957, 1968, and 2009) were preceded by La Niña conditions in the equatorial Pacific.
We have no basis yet to say whether SARS or MERS follow the same pattern, because those epidemics didn't last long enough. The last La Niña occurred in 2017–2018, and it was weak, which may be why the 2019–2020 influenza season was less severe than usual.
So there you have it: kill all the birds and buy a humidifier. Maybe all those windmills are good for something after all.
Update, Apr 06 2020 A preprint on MedRxiv[7] supports the idea that humidity strongly affects COVID-19 transmissibility. Of note, Mark Lipsitch is quoted in The Guardian as saying:
“Based on our best estimates from other coronaviruses, summer alone is not going to bring transmission to a level where the number of cases shrinks. It's just going to grow more slowly. . . . There's no question that coronaviruses are capable of transmitting in hotter, humid climates.”
The press jumped on this to claim that heat and humidity are not important. But if the transmission rate is lower, as Lipsitch said, fewer people will be infected, and this is what we've seen in Africa, South America, and Australia.
The evidence in the MedRxiv article shows that the case rate has not been uniform in latitude, but significantly higher in latitudes north of 30° but almost zero between 0° and 25°. Only as temperatures began to fall in the southern hemisphere did case rates begin to increase below 35° south latitude. Multiple linear regression confirmed strong correlation with temperature, with a p-value of 2×10−16.
Update, Apr 10 2020 A preprint on MedRxiv[13] provides more evidence of airborne transmission of SARS-COV-2. We should expect that the amount of airborne transmission would depend on the evaporation rate of droplets, and thus the humidity, so there will probably be a lot of variability in these experiments.
1. Cohen J (2020). Why do dozens of diseases wax and wane with the seasons and will COVID-19? Science, Mar. 13, 2020. doi:10.1126/science.abb7234 Link
2. Yang W, Lipsitch M, Shaman J. (2015). inference of seasonal and pandemic influenza transmission dynamics. Proc Natl Acad Sci U S A. 112, 2723–2728. doi: 10.1073/pnas.1415012112. PMID: 25730851 Link
3. Shaman J, Kohn M (2009). Absolute humidity modulates influenza survival, transmission, and seasonality. Proc Natl Acad Sci U S A. 106(9), 3243–3248. doi: 10.1073/pnas.0806852106. PMID: 19204283 PMCID: PMC2651255
4. Harper GJ (1961) Airborne micro-organisms: survival tests with four viurses. J. Hyg. Cambridge 59,479–486. doi:10.1017/S0022172400039176
5. Marr LC, Tang JW, Van Mullekom J, Lakdawala SS (2019). Mechanistic insights into the effect of humidity on airborne influenza virus survival, transmission and incidence. J R Soc Interface. 16(150), 20180298. doi: 10.1098/rsif.2018.0298. PMID: 30958176 PMCID: PMC6364647
6. Vanderbeke L, Spriet I, Breynaert C, Rijnders BJ, Verweij PE, Wauters J. Invasive pulmonary aspergillosis complicating severe influenza: epidemiology, diagnosis and treatment. Curr Opin Infect Dis. 31, 471–480. doi: 10.1097/QCO.0000000000000504. PMID: 30299367
7. Shaman J, Lipsitch M. (2013). The El Nino-Southern Oscillation (ENSO)-pandemic influenza connection: coincident or causal? Proc Natl Acad Sci U S A. 110 Suppl 1, 3689–3691. doi: 10.1073/pnas.1107485109. Epub 2012 Jan 17. PMID: 22308322 Link
8. Sun Z, Thilakavathy K, Kumar SS, He G, Liu SV (2020). Potential Factors Influencing Repeated SARS Outbreaks in China. Int J Environ Res Public Health. Mar 3; 17(5). pii: E1633. doi: 10.3390/ijerph17051633. PMID: 32138266 PMCID: PMC7084229
9. Moriyama M, Hugentobler WJ, Iwasaki A (2020). Seasonality of Respiratory Viral Infections. Annu Rev Virol. Mar 20. doi: 10.1146/annurev-virology-012420-022445. PMID: 32196426
10. Cheng YH, Wang CH, You SH, Hsieh NH, Chen WY, Chio CP, Liao CM (2016). Assessing coughing-induced influenza droplet transmission and implications for infection risk control. Epidemiol Infect. 144(2), 333–345. doi: 10.1017/S0950268815001739. PMID: 26211781
11. Li S, Eisenberg JN, Spicknall IH, Koopman JS (2009). Dynamics and control of infections transmitted from person to person through the environment. Am J Epidemiol. 170(2), 257–265. doi: 10.1093/aje/kwp116. PMID: 19474071
12. Triplett M (2020). Evidence that higher temperatures are associated with lower incidence of COVID-19 in pandemic state, cumulative cases reported up to March 27, 2020 https://www.medrxiv.org/content/10.1101/2020.04.02.20051524v1 doi: https://doi.org/10.1101/2020.04.02.20051524 s Link
13. Joshua L Santarpia, Danielle N Rivera, Vicki Herrera, M. Jane Morwitzer, Hannah Creager, George W. Santarpia, Kevin K Crown, David Brett-Major, Elizabeth Schnaubelt, M. Jana Broadhurst, James V. Lawler, St. Patrick Reid, John J. Lowe (2020). Transmission Potential of SARS-CoV-2 in Viral Shedding Observed at the University of Nebraska Medical Center https://www.medrxiv.org/content/10.1101/2020.03.23.20039446v2 doi: https://doi.org/10.1101/2020.03.23.20039446
apr 01 2020, 10:17 am. updated 6:13 pm. edited apr 02 2020, 7:01 pm graph added apr 04 2020, 1:46 pm. last updated apr 10 2020, 5:25 am
