There are now several reports of clinical trial results on pharmacological treatments of COVID-19 (Wuhan coronavirus). These results and those of ongoing trials will be important, but what we really need is a system for starting trials rapidly while these outbreaks are in progress.
Please note that many of the reports cited here are based on preprints that have not been peer-reviewed. (We're peer-reviewing them for you.) Some of the others are undergoing post-publication review and could even be retracted. Where the articles made statistical errors or had flawed experimental design, this is noted.
1. The French trial of hydroxychloroquine (HCQ)[1]
Subject population This study had a very unbalanced population. They started with 26 treated patients and 16 controls. Controls were not healthy volunteers, but infected patients who were not treated. Six in the treated group dropped out before the trial completed. Three of these went to ICU, one died, one stopped taking drug after three days because symptom free, and one stopped due to an adverse event (AE) of nausea. These six were dropped from the results.
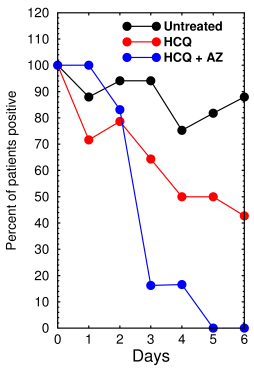
French trial results (redrawn from Gautret et al [1])
Of those who made it through the trial, 16% were initially asymptomatic, 61.2% had upper respiratory tract infection (rhinitis, pharyngitis, or low-grade fever and myalgia), and 22.1% had a lower respiratory tract infection (pneumonia or bronchitis). Average age for treated patients was 51.2 ± 18.7 (SD); controls were aged 37.3 ± 24. Treated patients were all from Marseille. Controls were from four regions in the south of France. Patients were not assigned randomly but allowed to choose which group they were in (treated or control). Patients that matched the authors' exclusion criteria were put in the control group. There was no placebo group, no uninfected control group, and no azithromycin (AZ)-only group.
Treatment Treatment was HCQ sulfate 200 mg 3x/day for 10 days. AZ was given to prevent bacterial infection and was not part of the original design.
Primary endpoint The authors' primary endpoint was virological clearance at day 6. Outcome measure was a non-quantitative, RT-PCR result of virus RNA in nasopharyngeal samples classified as either positive or negative.
Results No deaths were reported in the untreated group. By day 6, 8/20 treated with HCQ were negative, 6/6 treated with HCQ + AZ were negative, 2/16 untreated were negative. But note, this does not include the six who were dropped. The p-value was <0.001 by chi square (Fisher's exact test).
Comments It is well established that CQ and HCQ inhibit infection in cultured cells (see here). However, the study was underpowered, unrandomized, and unbalanced. The control group was from a different geographical region and markedly different age than the treated group, which makes it of dubious value as a control. The combination HCQ+AZ was the only treatment that was statistically significant. If the six that died, went to ICU, or were dropped had been included in the results, as they should have been, the results would likely not have been significant. The results are very hard to interpret and further research is needed.
Statistics Here are my re-calculations of their 6-day results using the Fisher's chi square test:
| Group | Description | Uninfected | Infected | P value vs. Con | P value vs. HCQ |
|---|---|---|---|---|---|
| 1 | Control | 2 | 14 | ||
| 2 | HCQ | 8 | 12 | 0.132 | |
| 3 | HCQ+AZ | 6 | 0 | 0.0004 | 0.017 |
| 4 | HCQ + HCQ+AZ | 14 | 12 | 0.0096 | 0.388 |
| 5 | HCQ + excluded | 8 | 18 | 0.2696 | |
| 6 | All HCQ + excluded | 14 | 18 | 0.0501 |
This is what the table shows. Groups 4, 5, and 6 were not in the paper.
- The HCQ group (Group 2) is not significantly different from the untreated controls.
- The HCQ+AZ group (Group 3, green) is highly significantly different from the control group and significantly different from the HCQ-only group.
- If all the subjects given HCQ or HCQ+AZ are combined (=Group 4) the result is statistically significant at p<0.01.
- If the six excluded subjects were all virus-positive and included in the HCQ group (Group 5), the change would be non-significant.
- If the subjects receiving HCQ or HCQ+AZ and the 6 excluded subjects were all combined (Group 6), the effect would be statistically significant if any of the 6 of the excluded ones were virus-free.
Conclusion: HCQ alone did not show an effect. The combination of HCQ and AZ looks promising, but better control groups and bigger populations are needed. We also need data on how age affects the virus clearance rate.
Update (03/28/2020) The French group published a new report[10] saying that 80 patients with COVID-19 were treated with HCQ + AZ. It was not a clinical trial and there was no control group. All patients got 200 mg HCQ 3x/day for 10 days plus AZ 500 mg on day 1 followed by 250 mg/day for the next 4 days. The authors say patients should be hospitalized before treatment if they have an underlying heart condition and the ECGs should be monitored.
Results Virus RNA decreased over time. The authors suggest that the decrease is faster than reported by Chinese patients in previous reports. The authors deserve praise for their dedication in treating their patients but this study does not evaluate or demonstrate the effectiveness of the treatment. There are reports that a clinical trial is being started next week in New York.
2. The Chinese hydroxychloroquine study
In a brief report from Zhejiang University[11], 15 patients were given hydroxychloroquine (400 mg, 1x/day) and 15 were used as controls.
- By day 7, 13 patients in the treated group (86.7%) and 14 in the control group (93.3%) tested negative for viral RNA. (Not significant)
- Test group became negative for virus RNA 4 (1–9) days and control group became negative 2 days (1–4) after admission. (Not significant)
- All of the subjects had a mild case at enrollment.
- All subjects were also given interferon-α. Eighty percent of treated patients and 2/3 of controls also received Arbidol (阿比多尔), an antiviral also known as umifenovir (NOT Abidol (阿比多), as a well known computer online mistranslator says—this is a narcotic and the use of an online translator by some bloggers is causing widespread confusion). Two patients also got lopinavir / ritonavir (antiretrovirals).
- Temperature returned to normal by 4 days after admission.
- There were no statistically significant differences in symptoms or virus between the groups.
- Excluding patient drop-out, as many as 900 patients would be needed to see an effect.
The authors say HCQ effects might be easier to see in more severely ill patients. They also say that the virulence of SARS-CoV-2 seems to be decreasing. In the discussion they say that due to the fact that the patients' condition changes so rapidly, a randomized controlled study will be essential. The fact that their patients were stuffed to the gills with antivirals and interferon means the negative HCQ results may not be 100% conclusive.
Comment If any of these other drugs touch on a pathway affected by HCQ it would create a ceiling effect that would make the results of this trial meaningless.
3. The unpublished Chinese trials of chloroquine phosphate[2]
A brief report from China mentions that 15 clinical trials of chloroquine or or hydroxychloroquine were done in China in >100 patients. The authors claim that chloroquine phosphate was “superior to the control treatment in inhibiting the exacerbation of pneumonia, improving lung imaging findings, promoting a virus-negative conversion, and shortening the disease course.” I have not yet been able to find the actual results and no data are presented in the article. The only sources given are to a news conference by the Chinese government. Until the results are published there is no way to evaluate their accuracy. q
Update There seems to be confusion on the internet about chloroquine phosphate. Chloroquine is basic (pKa 8.4 and 10.1) and therefore it's usually sold as a salt, which means it's been neutralized by sulfuric, hydrochloric, or phosphoric acid. This means chloroquine phosphate, chloroquine sulfate, and chloroquine hydrochloride all contain the same ingredient.
4. The Chinese study of lopinavir and ritonavir[3]
This was a randomized controlled open-label trial of two antiviral drugs.
Subject population 99 treated, 100 controls. Some subjects also received a variety of other drugs including vasopressors, ventilation, antibiotics, and glucocorticoids. Enrollment ended when remdesivir, a better drug which prevents MERS-CoV infection in rhesus macaques, became available. Even 8 years after MERS-CoV, there are few treatment options for MERS.
Treatment Lopinavir (400 mg) and ritonavir (100 mg) 2x/day for 14 days.
Primary endpoint The end point was time to clinical improvement, which the authors defined as the time from randomization to either improvement of 2 points on a 7 category scale, or discharge from the hospital. The 7-category scale was: 1=resumed normal activities, 2=not hospitalized but unable to resume normal activities, 3=hospitalized not requiring O2, 4=hospitalized requiring O2, 5=hospitalized with nasal high-flow oxygen or ventilation, 6=hospitalized with ECMO (extracorporeal membrane oxygenation) or invasive ventilation, 7=death.
Results No effect. The authors stopped recruiting patients when they realized they were testing the wrong drug. The viral load, measured by RT-PCR, decreased to 0 by day 21 in both controls and treated patients. There was 19.2% fatality in the treated and 25% fatality in controls. The difference was not statistically significant. All deaths were due to respiratory failure. Treatment was stopped in 13 subjects due to adverse events.
Comments This trial appears to have been carried out properly. Viral load was reported in log10 copies per ml. At day 1 it was 4.0±2.1.
5. Tocilizumab
A report in a tabloid newspaper in the UK claimed that Chinese researchers gave tocilizumab to 20 COVID-19 patients and that 19 were discharged within 14 days. This is roughly the same period by which the virus is cleared without treatment. The results were published on the non-peer-reviewed site ChinaXiv and so the credibility of the claim, assuming for the sake of argument that the newspaper reported it accurately, remains to be established.
Tocilizumab (Actemra) is a monoclonal antibody approved in the USA for systemic juvenile idiopathic arthritis (a type of arthritis that occurs following a fever), rheumatoid arthritis, giant cell arteritis, and CAR-T cell induced cytokine release syndrome, aka cytokine storm. There are uncontrolled studies supporting its use in adult onset Still disease, familial Mediterranean fever, TRAPS, mevalonate kinase deficiency, and AA amyloidosis, all of which are autoinflammatory diseases[9]. It binds to the interleukin-6 receptor and many adverse events have been reported[8] but it remains a promising treatment for cytokine storm.
6. The Chinese retrospective study of Arbidol [12]
Treatment Lopinavir (400 mg) + ritonavir (100 mg) (=LPV/r) every 12 hr with or without Arbidol 200 mg every 8h. Treatment continued from 5–21 days until virus RNA was negative by RT-PCR three times. 73% also received immunoglobulin therapy, which is used to reduce cytokine storm, and 61% also received antibacterial therapy.
Subject population 33 patients aged 44–56. 16 got Arbidol + LPV/r ('combination' group), 17 got LPV/r alone ('monotherapy' group). For ethical reasons no placebo group was used.
End point Negative conversion rate of SARS-CoV-2
Results Arbidol significantly increased the rate of virus clearance.
| Group | Uninfected | Infected | P value |
|---|---|---|---|
| Monotherapy 7d | 6 | 11 | 0.037 |
| Combination 7d | 12 | 4 | |
| Monotherapy 14d | 9 | 8 | 0.0167 |
| Combination 14d | 15 | 1 |
69% of the patients had elevated bilirubin and 43.7% experienced diarrhea and nausea but no subjects dropped out due to the AEs.
Comment Arbidol is an antiviral used in Russia and China against influenza-type viruses. It is said to be more effective against RNA viruses than DNA viruses. Chinese doctors often use it in combination with interferon-alpha[11] which is part of the innate immune system that attacks viruses nonspecifically. Arbidol is said to increase interferon release. Interferon is reported to restore the ability of Treg cells to regulate immune cells by reducing levels of IL-12[14] but can induce major depressive disorder.[13]
7. The Renmin randomized trial of hydroxychloroquine [14]
Researchers at the Renmin (People's) Hospital of Wuhan University posted a non-peer-reviewed preprint on medRxiv.
Subject population 62 COVID-19 patients were randomly assigned into two groups. They were randomly assigned to treated and control groups.
Treatment Treated group received 200 mg HCQ 2x/day for 5 days. Both groups got antibiotics, antivirals, corticosteroids, oxygen, plus immunoglobulin to inhibit cytokine storm.
Results
Fever
| Group | N | Days of fever | P value |
|---|---|---|---|
| HCQ | 22 | 2.2 ± 0.4 | 0.00155 |
| Control | 17 | 3.2 ± 1.3 |
The p-value shown here (0.00155) differs by a factor of 2 from the statistic printed in the paper (0.0008), suggesting they may have used a one-tailed test by mistake. Either number indicates a highly significant effect on fever.
Cough
| Group | N | Cough | P value |
|---|---|---|---|
| HCQ | 22 | 2.0 ± 0.2 | 0.0016 |
| Control | 15 | 3.1 ± 1.5 |
Disease progression
| Group | Progressed | No progression | P value |
|---|---|---|---|
| HCQ | 0 | 31 | 0.113 |
| Control | 4 | 27 |
Pneumonia
| Group | Exacerbated | Unchanged | Improved | P value |
|---|---|---|---|---|
| HCQ | 2 | 4 | 25 | 0.047609 |
| Control | 9 | 5 | 17 |
Results HCQ significantly reduced the cough and days of fever. It had no effect on the progression of the disease. The p=0.0476 under ‘Pneumonia’ is barely statistically significant. It appears that HCQ may cause slight improvement in pneumonia.
Conclusion HCQ reduced fever and cough. The important parameter, virus load, was not reported, so this study, while encouraging, is flawed. According to the methods section, the authors have access to a RT-PCR machine but they did not report any results, suggesting that they got an unwanted result.
As with the other study above, if any of the antivirals touched on a pathway affected by HCQ it would create a ceiling effect that would obscure any results from this trial. The near absence of disease progression in both groups suggests that this may be happening.
Update, Apr 04 2020
8. The Molina et al. study of Hydroxychloroquine / Azithromycin [16]
Treatment HCQ (600 mg/d for 10d) + AZ (500 mg day 1, 250 mg days 2-5), same as in Gautret et al. Treatment was stopped in one patient on day 4.
Subject population 11 subjects, 8 of whom had significant comorbidities (2 obesity, 5 cancer, 1 HIV) known to produce poor outcomes.
End point This was not a controlled trial and no end point was stated.
Results Within 5 days, 1 died, 2 went to ICU. Virus measured by RT-PCR was still positive at day 5–6 in 8 the 10 surviving patients.
Comment No control group and no statistics. There was insufficient data in this brief report to be sure what really happened. Authors don't mention whether the five cancer patients were being treated for their cancer. Was the HIV patient being treated? What were the actual PCR results? What about their fever? Which patients died or went to ICU? The results in this study are nearly uninterpretable. The paper is full of disparaging comments about other studies, suggesting a lack of objectivity. From the study design, it does appear that the authors stacked the deck in favor of their conclusion.
Update, Apr 11 2020
9. The Grein et al. expanded access study of Remdesivir at Gilead Sciences and comparison of ventilators vs noninvasive oxygen support[17]
Treatment Remdesivir (200 mg for 1 day then 100 mg for 9 days).
Subject population 61 subjects, 53 completed treatment.
End point This was not a controlled trial and no end point was defined.
Results Seven died (13%) including 6/34 (18%) receiving invasive ventilation and 1/19 (5%) receiving noninvasive oxygen support. The differences were not statistically significant (HR 2.78, 95% CI = 0.33 to 23.19). Patients >70 years of age were more vulnerable (HR 11.34, 95% CI = 1.22 to 2.99).
At 28 day follow-up, 8/34 (24%) of patients receiving invasive ventilation and 17/19 (89%) receiving noninvasive oxygen had been discharged. 10/10 (100%) of those receiving low-flow oxygen were discharged.
Comment Update 04/17/2020 The news media are strongly hyping this study today after Gilead made a positive statement in Stat News claiming that 123/125 patients in a later study survived. So far no actual data are available.
This earlier report, published in NEJM, doesn't show anything about the efficacy of remdesivir because there was no control group. The only meaningful result is that invasive ventilation does not increase mortality but appears to prolong recovery times. No mention of any benefits from Remdesivir other than to say that “clinical improvement was observed in 36 of 53 patients (68%).” No measurements of viral load, fever, or disease severity were made. Over half had signs of liver toxicity.
The authors say “Measurement of efficacy will require ongoing randomized, placebo- controlled trials of remdesivir therapy.”
To a layman, it may seem understandable that no one would volunteer to be in a control group, but they could have been offered alternative treatments such as lopinavir/ritonavir, the anti-AIDS antivirals. Without a control group there is no way to compare the severity of disease and standard of care treatment in different locations. Many tens of thousands of other patients across the globe are also not getting remdesivir, so there's no excuse for not including one. Indeed, by the authors' logic, it would be unethical not to give every patient every drug.
Update, May 26 2020: This study has been found to have serious methodological errors.
10. The third French study of HCQ + AZ (Raoult et al., unpublished)
Treatment Hydroxychloroquine and azithromycin (dosage not stated).
Subject population 1061 subjects, mostly with clinical classification of 0–4 (low)
End point This was not a controlled trial and no end point was defined.
Results 10 went to ICU, 5 died, and 973 cleared the virus by day 10. Patients with hypertension, diabetes, cancer, or coronary artery disease and patients on angiotensin II receptor blockers or beta blockers had significantly worse outcome.
Comment No control group and no way to assess efficacy. Not a complete paper, table and abstract only.
11. The Zhou et al. study of interferon-α2b and Arbidol (Tongji Medical College, Wuhan) [18]
Treatment Nebulized IFN-α2b, 5 mU bid) (7/77), arbidol (200 mg tid)(24/77), or both (46/77). 24 of the last group received continued IFN after arbidol was stopped. All patients also received antibiotics. Fever was suppressed with ibuprofen.
Subject population 77 subjects, unbalanced and not randomized, all moderate cases. The Arbidol-only group had more severe disease and higher comorbidities at the start.
End point Negative viral load was defined as a decrease in viral RNA measured by RT-PCR. No untreated controls.
Results IFN significantly shortened the time to viral clearance (p=0.003). IFN subjects also maintained low IL-6 levels throughout treatment indicating lower inflammation. Arbidol-only patients had roughly 10× higher IL-6 (p=0.0033). No subj required intubation or O2 supplentation.
Comment The authors recommend a randomized controlled trial IFN or tocilizumab.
Update, Apr 14 2020
12. The Mahevas et al. (Paris) study of hydroxychloroquinone [19]
Treatment 84 received HCQ 600 mg/day in the first 48h after hospitalization, 97 received HCQ but delayed for at least 48h. Not randomized, but used a complex formula based on comorbidities to balance the groups. Used robust statistics. 17 in HCQ group also got azithromyzin. 64 in HCQ group also got amoxicillin + clavulanic acid.
Subject population 181 patients with SARS-CoV-2 pneumonia, balanced delayed-treatment controlled study
End point Transfer to ICU, death, or discharge within 7 days
Results
| Group | ICU or died | Not ICU+alive | P value | ARDS | No ARDS | P value | |
|---|---|---|---|---|---|---|---|
| HCQ | 16 (20.2%) | 68 | 0.714 | 24 | 60 | 0.50 | |
| Control | 21 (22.1%) | 76 | 23 | 74 |
| Group | Died | Alive | P value |
|---|---|---|---|
| HCQ | 3 | 81 | 1.000 |
| Control | 4 | 93 |
Note, 7 in HCQ group had corrected QTc prolongation, 1 HCQ had uncorrected AV block, so 8 were dropped after 3–9 days.
ARDS = acute respiratory distress syndrome by day 7
Comment This study shows that delaying HCQ for 2 days has no effect on ARDS or referral to ICU. Note that the controls also got HCQ starting at 48 hour or so after admission and continuing for the rest of the 7 day period. Virus load, pneumonia, fever, and time to recovery were not reported. A well controlled study but the only criterion was whether subjects were sent to ICU, which is a subjective judgment.
The authors say that timing of treatment is critical and that measuring virus load is not as clinically relevant as counting ICU admissions. This is disputable. They also say HCQ blocks hERG. If so, this would mean HCQ would never be considered for FDA approval if submitted today.
13. The Zhang et al. comparison of hypertension treatments and chloroquine (Wuhan & Beijing) [20]
Treatment Retrospective study of 487 COVID-19 patients being treated with calcium-channel blockers and other drugs for hypertension. The authors included some in vitro results on chloroquine as well.
End point Survival rate
Results
| Group | Survived | Died | P value vs untreat | P value vs Non-CCB |
|---|---|---|---|---|
| No treatment | 7 | 2 | – | 0.334 |
| Amlodipine | 41 | 3 | 0.15 | 0.0012 |
| Nifedipine | 14 | 2 | 0.52 | 0.065 |
| Other CCB | 3 | 1 | 0.91 | 0.548 |
| Non-CCB drug | 10 | 7 | 0.22 | – |
| Any CCB | 58 | 6 | 0.25 | 0.0015 |
| Non-CCB drug | 10 | 7 | 0.22 | – |
Above is a re-analysis of their Table using 2×2 Chi-square test w/o Yates correction. Using Fisher's exact test, the numbers in green are 0.0031 and 0.0044, which is still highly significant.
"Non-CCB drug" includes Angiotensin II receptor blockers, ACE inhibitors, β-blockers, and thiazide. Amlodipine or “any calcium channel blocker” reduced mortality compared to other classes of anti-hypertension drugs. No drugs had a statistically significant effect compared with untreated patients due to the small numbers of patients. Note that mortality tended to increase with non-CCB drugs.
Survival increased from 58.8% on a non-CBB hypertensive drug to 90.6% with a CBB and 93.2 with amlodipine.
Hypertension is a major risk factor for COVID-19. Angiotensin receptor blockers and ACE inhibitors, which are used to treat hypertension, are known to induce ACE2, which is the receptor for SARS-CoV-2 virus. Thus it is predicted that these drugs would increase the severity of the disease. This important study supports the theory, as does the finding that nicotine protects against the virus, possibly by reducing ACE2 levels. However, several other studies have found no harmful effect of ACE inhibitors. More research is needed.
Update, May 15 2020 Several studies have now been done on this hypothesis. Most but not all have found that there is little or no risk to ACE inhibitors. Most physicians would recommend not changing your blood pressure medication.
Update, Apr 17 2020
14. The Tang et al. open label randomized trial of a high dose of hydroxychloroquine (Shanghai) [21]
Subject population 150 patients hospitalized with COVID-19.
Treatment HCQ 1200 mg daily for 3d followed by 800 mg daily for remainder of the 2 to 3 week trial. 75 got HCQ + SOC and 75 got SOC alone. SOC = standard of care for COVID-19, which included antiviral agents including lopinavir-ritonavir, arbidol, oseltamivir, virazole, entecavir, ganciclovir and/or interferon-alpha.
End point 28-day conversion rate (virus measured by RT-PCR)
Results No effect on virus or time to alleviation of clinical symptoms. HCQ increased the rate of symptom alleviation during week 2 but not week 1 and 3. Effect on symptoms was bigger when corrected for the effect of antivirals. HCQ increased the rate of recovery of lymphocytopenia and CRP, which suggests an effect on inflammation. 30% of HCQ patients had adverse events, mostly diarrhea.
Comment HCQ did not work on the virus, but seemed to benefit inflammation and a higher rate of symptom alleviation. Only two patients got azithromycin, so maybe AZ is required for its effect. No cardiac AEs.
15. The Liu et al. retrospective study of Arbidol [22]
Subject population 504 patients hospitalized with COVID-19 from three different hospitals.
Treatment Compared different antiviral drugs including Arbidol (257 patients), Oseltamivir (66), and Lopinavir/Ritonavir (259). Some of the patients also got glucocorticoids, immunoglobin, oxygen, albumin, and ventilation. The percentage of patients receiving these other treatments was different in the three hospitals, as was the percentage receiving each antiviral.
End point Mortality and lesion absorption determined from chest CT scan.
Results
| Group | % died |
|---|---|
| Arbidol | 7.0 |
| No Arbidol* | 24.7 |
| Oseltamivir | 12.1 |
| No Oseltamivir* | 16.2 |
| Lopinavir/Ritonavir | 14.3 |
| No Lop/Rit* | 17.1 |
The authors also say the average lesion size is smaller in all three antivirals, but data are not supplied. * Meaning is unclear
Comment The figures referred to in the paper are not actually in the manuscript so there is no way to evaluate the claimed results. Treatment of the control group was not specified. No data on the other drugs referred to in the paper. These factors would cause a peer-reviewer to recommend a hard rejection.
16. The Meng et al. open-label test of interferon nasal drops on medical staff [23]
Subject population 2944 medical staff in Shiyan City and Wuhan.
Treatment Medical staff in Shiyan City, Hubei received interferon. Medical staff in Wuhan were untreated controls.
End point New-onset COVID-19 within 28 days.
Results Staff in Hubei had a total of 474 confirmed cases, 41 severe cases, and 2 deaths. Staff in Wuhan had a total of 1330 confirmed cases, 191 severe cases, and 3 deaths.
Comment Nearly impossible to discern what happened or how severity was assessed. Authors claim that interferon reduced the incidence of infection.
Update, Apr 21 2020
17. The Magagnoli et al. Veterans Administration retrospective analysis of hydroxychloroquine (U. South Carolina) [24]
Subject population Records of 368 male patients from all VA medical centers between March 9 and April 11 2020 were combined and analyzed retrospectively.
Treatment 97 = HCQ, 113 = HCQ + azithromycin, 158 = no HCQ. Dosage and treatment length was not stated. 31.5% of the control group also got azithromycin. Between 9 and 17% got ACE inhibitors or Angiotensin II blockers. No significant difference among the three groups for these two.
End point Death/discharge and ventilator rate
Results
| Group | Deaths | Discharge | % deaths | P-value | Ventilator | No vent. | P-value | |
|---|---|---|---|---|---|---|---|---|
| HCQ | 27 | 70 | 27.8 | 0.00308 | 12 | 78 | 0.185 | |
| HCQ + AZ | 25 | 88 | 22.1 | 7 | 94 | |||
| Untreated | 18 | 140 | 11.4 | 25 | 152 | |||
| All HCQ | 52 | 158 | 24.8 | 0.00120 | 19 | 172 | 0.261 | |
| Untreated | 18 | 140 | 11.4 | 25 | 152 |
The above statistics were re-analyzed by Chi Square Test from the authors' data. The "All HCQ" includes HCQ and HCQ+AZ and was analyzed by Fisher's Exact test.
Comment The baseline death rate was 11.4% of all patients, an unusually high number. Two-thirds of the patients were black males. There was no significant difference in deaths between HCQ and HCQ+AZ, but HCQ vs untreated was highly significantly worse. No conclusion can be drawn about any benefits or risks from AZ, as some "untreated" patients also got AZ.
Due to the very high baseline death rate, this trial shows that HCQ may increase death rates in seriously ill patients. Death rates were not separated by race so there is no information on whether HCQ is more harmful to one race or another. Baseline and final virus RNA, fever, and disease severity were not reported.
IMPORTANT NOTE The news media are hyping this as a new trial showing that HCQ is harming patients.
However, this was not a randomized controlled trial and there is a strong suggestion that HCQ might have been preferentially given to sicker patients. This is shown by the clinical chemistry results in the authors' Table 2, which showed significant baseline differences among the three groups for pulse oximetry, ALT, AST, serum albumin, bilirubin, RBCs, hematocrit, C-reactive protein, Troponin I, leukocytes, lymphocytes, and platelet count.
If we calculate the statistics on just the pulse oximetry from their Table 2, we can see that there's a significant difference among the three groups. Most of this is in the HCQ+AZ group, which was more hypoxic at the start of the trial. The percentage of patients who were hypoxic was 40% higher in the HCQ group and 60% higher in the HCQ+AZ group compared to the controls, indicating that the patients who were treated started with more severe illness.
Pulse Oximetry baseline SpO2
| Group | 75–94 | ≥95 | P-value | Pct hypoxic |
|---|---|---|---|---|
| HCQ | 36 | 61 | 0.019 | 37.1 |
| HCQ+AZ | 48 | 65 | 42.5 | |
| Untreated | 42 | 116 | 26.5 |
No conclusion about HCQ can be derived from the data presented. If I were peer-reviewing this one, I'd recommend rejection.
Update, Apr 25 2020
18. The Brazil study of chloroquine [25]
Chloroquine is known to be more toxic than hydroxychloroquine. A recent study [26] recommended loading doses of 30 mg/kg over 48h or 70 mg/kg over 5 days for adults (14 mg/kg/day), which has proved safe and effective for malaria. Chloroquine has a narrow safety margin and three times the therapeutic dose can be lethal.
In this study, Barba et al. [26] ran a trial testing high dose chloroquine on severely ill patients. The main purpose was to test its toxicity. The authors planned to continue treatment for 28 days but their hospital's independent safety board stopped this experiment on day 13.
Subject population 81 severely ill adults with COVID-19, 2 doses of chloroquine phosphate. Most of the patients were already on oxygen therapy. It is not stated whether this means ventilation or not. More older patients with heart disease were assigned to the high-dose group than the low-dose group.
Treatment High dose CQ (600 mg CQ twice a day for 10 days) or low-dose CQ (450 mg twice a day on day 1 and once daily for 4 days). No control groups. Patients also received ceftrixone (i.v.), azithromycin, and Oseltamivir.
End point Patient lethality
Results
| Group | Died | Survived | P-value | Cardiotox | No tox | P-value | |
|---|---|---|---|---|---|---|---|
| CQ high | 16 | 25 | 0.026 | 7 | 30 | 0.515 | |
| CQ low | 6 | 34 | 4 | 32 |
The statistics above were calculated from the authors' data using Fisher's Exact test. Cardiotoxicity was defined as QTcF > 500 ms.
Patients on high dose had significantly higher death rate. Cardiotoxicity was not statistically significant among the two groups. Two patients in the high dose had ventricular tachycardia.
Comment It is not clear why anyone would test chloroquine and not HCQ, which is known to be safer. A total of 12,000 mg was given over 10 days. The authors say this dose was selected based on recommendations from the Guangdong Province Health Commission. A previous trial of this dose had been done on cancer patients with good safety.Patient body weight was not reported and authors did not calculate CQ dose by body weight. The lightest patient weighed 50 kg. Assuming their weights ranged between 50 and 70 kg, the high dose would be 172 to 240 mg/kg over 10 days. This is 22–70% higher than the safe daily dose used for malaria and more than twice as much total drug. This suggests that CQ continued for longer than recommended may cause toxicity.
It is of note that in the Tang et al. study (described above), 14,800 mg of HCQ was given over 14 days with no reported drug-induced deaths. Table 2 of the current study shows no statistically significant differences in five measures of toxicity (Hb, creatinine, CK, CKMB, and QTcF), so the authors concluded that the deaths were due to Covid-19, not the treatment. The authors say the death rate is not atypical for Covid-19, but note that oseltamivir also raises the QTc interval, suggesting a possible drug interaction.
The authors recommended that randomized trials be done (presumably at more reasonable doses) to test the efficacy of CD in patients with mild disease. However, they certainly knew that health agencies would use this study to recommend against using CQ at all, and that an RCT is now unlikely.
When testing a drug of known toxicity it would be a good idea to measure the body weight of the patients and give doses relative to their weight, as has been routinely done for decades in animal experiments. This would make clinical trial dosing more reliable and safer.
Update, Apr 30 2020
19. The Beijing study of remdesivir [27]
Finally a randomized placebo-controlled trial of remdesivir, published in Lancet.
Subject population 237 patients randomly assigned, 158 = remdesivir and 79 = placebo.
Treatment Remdesivir i.v. 200 mg on day 1, then 100 mg/day on day 2–10 by infusion, or placebo infusion. Subjects also got lopinavir-ritonavir (28/29%) (treated/placebo), interferons (29/38%), antibiotics (90/94%) and corticosteroids (65/68%). Most were given oxygen (82%/83%), and 18%/12% required non-invasive ventilation.
End point Time to clinical improvement on day 28, defined as a decrease by two levels on a scale of 1 (discharged) to 6 (death).
Results
| Group | Total died | Survived | P-value | Improved | Not improved | P-value | |
|---|---|---|---|---|---|---|---|
| Remdesivir | 22 | 136 | 1.00 | 103 | 45 | 0.317 | |
| Placebo | 10 | 68 | 55 | 33 |
| Group | Died ≤10d | Survived | P-value | Died >10d | Survived | P-value | |
|---|---|---|---|---|---|---|---|
| Remdesivir | 8 | 63 | 0.583 | 12 | 72 | 0.756 | |
| Placebo | 7 | 40 | 3 | 28 |
Statistics recalculated from authors' data by Fisher's exact test.
No statistically significant change in early, late, or overall death rate or in clinical improvement. Clear-cut negative result. Adverse events occurred in 66% of remdesivir and 64% of placebos. Remdesivir was stopped early due to adverse events in 18 (12%) treated and 4 (15%) controls.
Comment Groups were evenly balanced by sex, comorbidities, fever, and WBC, lymphocyte, and platelet counts. Very well done study.
Note Some other sources are saying this study is not credible, saying “confounders are huge” because the authors claim they couldn't find enough subjects and because it was funded by the Chinese government. This reflects suspicion of the PRC government's claim that COVID-19 no longer exists in Wuhan. However, whether this is true or not bears no relation to the statistical findings in the paper. It is relevant that other Chinese researchers abandoned lopinavir / ritonavir when remdesivir became available, discrediting claims of bias.
Update, May 02 2020
20. The retrospective Wuhan hydroxychloroquine study [28]
A retrospective study from Tongji Medical College in Wuhan has encouraging news about HCQ. Not a randomized controlled study, but the first positive result in a while for this drug.
Subject population 568 critically ill patients between Feb 1 and Apr 8, 2020. Results were adjusted for age, sex, oxygen saturation, baseline treatment drugs, and comorbidities including hypertension, coronary heart disease, and COPD. Populations did not significantly differ in any of these parameters.
Treatment Out of a total of 568 critically ill patients, all in the same hospital. 48 received hydroxychloroquine, 200 mg 2×/day for 7–10 days for a total of 4000 mg. This is a much lower dose than the widely reported Brazil study where researchers gave 12,000 mg (three times as much) of chloroquine (which is about 40% more toxic). Unlike the VA study, the dosage is specified.
Some patients also received lopinavir/ritonavir, entecavir, ribavirin, immunoglobulin, “immunoenhancers”, antibiotics, and interferon. The amounts of antibiotics and interferon different between groups. The authors tried to correct for comorbidities and different baseline treatments using Cox regression analysis. This did not change the results, which were highly significant.
Specifically, antibiotics in HCQ vs non-HCQ were 77.1% vs 89.4%, interferon was 0% vs. 10.4%, antivirals were 41.7% vs. 44.4%, IgG was 52.1% vs. 47.1%, and immunoenhancer was 16.7% vs 17.3%.
End point Survival.
Results
| Group | Total died | Survived | P-value |
|---|---|---|---|
| HCQ + other drugs | 9 | 39 | 0.0002 |
| Other drugs only | 238 | 282 |
Statistics recalculated from authors' data by Fisher's exact test. P-value was 0.000303 using different software.
Average hospital stay was not significantly changed. Interleukin-6 was also reduced by HCQ (before=22.2 pg/ml, after=5.2, p=0.002), but increased after HCQ was stopped. IL-6 was not significantly changed in the non-HCQ patients.
Comment The death rate in the non-HCQ group was extremely high (45.8%). The dosage of HCQ was less than Raoult's original HCQ study. The results are highly statistically significant and suggest that excessively high drug levels may be counterproductive.
If I were peer-reviewing it, I'd recommend minor revision. Mainly they should provide all the IL-6 results in a table so they can be examined more clearly. The error bars on their IL-6 graph aren't identified; presumably they are standard deviation.
Update, May 06 2020
21. The observational Guangdong chloroquine study [29]
A preliminary prospective observational study from Sun Yat-sen University in Zhuhai, Guangdong. Not a randomized controlled study, but the first positive result in a while for this drug.
Subject population Patients with moderate confirmed SARS-CoV-2 infection from 12 hospitals.
Treatment 197 got CQ, 176 historical controls. Chloroquine was 500 mg once or twice daily for ten days (total = 5000 or 10000 mg). The high dose is slightly lower than the Brazil study where researchers gave 12,000 mg and found large numbers of deaths. The difference may be that the patients here all had mild illness. In some tables they combined the data from high and low doses.
End point Time to achieve undetectable RNA
Results CQ reduced the time to undetectable viral RNA by 5 days. CQ also reduced the duration of fever. No beneficial effect on length of hospital stay or duration of oxygen. Authors also tested whether viral RNA reappeared in any patients after discharge and found only three. Low dose CQ worked as well as higher doses.
Incidence of AEs was 26.9% in CQ group and 32.4% in controls, mostly vomiting, nausea, and thirst. High dose CQ patients had more anxiety, dizziness, and sleep disorder indicating more neurological adverse events. No serious or unexpected AEs were reported.
| Group | −RNA day 10 | +RNA day 10 | P-value |
|---|---|---|---|
| CQ | 180 | 17 | <0.00001 |
| Control | 101 | 75 |
Statistics recalculated from authors' data by Fisher's exact test.
| Group | Duration of fever | N | P-value |
|---|---|---|---|
| CQ | 1.2 ± 0.64 | 197 | 0.0000103 |
| Control | 1.9 ± 2.09 | 176 |
Comment Not a controlled randomized study. Authors say chloroquine also had an antipyretic effect which contributed to the result. Chloroquine has a long half-life (20–60 days), indicating a cumulative effect. This means long duration of treatment will produce many more adverse events. The authors acknowledge many deficiencies in the study, including that differences in duration between symptom onset and recovery in the two groups could have affected the results, but say post-hoc analysis discounts it.
Update, May 08 2020
22. The Geleris observational hydroxychloroquine study [30]
Subject population 1376 patients followed up after an average of 22.5 days at Presby hospital in New York. The authors say patients who died, critically ill patients on ventilators, and patients who were discharged within 24h were excluded. The study was not balanced: HCQ patients were more severely ill than the untreated patients.
Treatment 811 got HCQ (600 mg twice on day 1, then 400 mg/day for 5 days, total 3200 mg). Some patients (60%) also received azithromycin 500 mg on day 1 then 250 mg/day for 4 more days. Some patients also received sarilumab, a monoclonal antibody against interleukin-6 receptor. 565 patients did not receive HCQ. 22% of these controls received AZ. 49.1% of the treated but only 6.7% of the untreated also had hypertension. The authors tried to correct for this by propensity-score matching. 74.5% of the treated and 54% of the untreated also got additional antibiotics.
End point Death or intubation
Results
| Group | Intubation or death | No intubation /death | P-value |
|---|---|---|---|
| HCQ | 262 | 549 | <0.0001 |
| No HCQ | 84 | 481 |
Statistics recalculated from authors' data by Fisher's exact test. The apparent increase in mortality is not real because the HCQ patients were more severely ill than the controls. When the authors corrected for the higher baseline disease severity in the HCQ group, the difference became non-significant.
Comment Another flawed study. Not a controlled randomized trial. No post-treatment rates of virus clearance, CRP levels, or other indices of inflammation are reported. Physicians were allowed to prescribe HCQ, azithromycin, both, or neither for each patient as they saw fit. The conclusions are hard to interpret because the results depend entirely on whether the statistical correction was appropriate.
The only conclusion one can draw is that HCQ is not a miraculous cure. The authors recognize this, saying “the study should not be taken to rule out either benefit or harm of hydroxychloroquine treatment. However, our findings do not support the use of hydroxychloroquine at present, outside randomized clinical trials testing its efficacy.”
It is curious that the news media have given this study extensive coverage, emphasizing the second sentence above, while other positive observational studies such as this one have been ignored.
Summary
While HCQ and CQ might be having an effect, it's hard to be sure because all the studies so far have been flawed. The general opinion seems to be that it doesn't hurt to give patients HCQ as its toxicity is low, but Dr Raoult's paper wouldn't have passed peer review under normal circumstances and could be retracted by the journal.
Antivirals also seem to have limited effect, with ribavirin, remdesivir, lopinavir — ritonavir, and Arbidol appearing to be less effective than hoped. So far the most promising treatment seems to be interferon.
There are other reports of antibiotics working against SARS-type viruses, so the azithromycin results shouldn't be dismissed out of hand. There's one report that teicoplanin, an antibiotic used for staphylococci, works against MERS in vitro. Chloroquine was found to be effective against SARS in vitro way back in 2004.[4,5] but I could find no published reports of any clinical trials on SARS other than for vaccines.
Muller et al.[6] expressed concern back in 2004 that despite thousands of patients receiving ribavirin and corticosteroids during the SARS outbreak, no clinical trials were conducted. They blame the 18-day bureaucratic delay in Canada for the loss of 60% of the SARS patients who could have been enrolled. These outbreaks are short-lived; finding enough patients to run a trial after the outbreak is over is very difficult. They wrote: “If a second global SARS outbreak occurred, clinicians would not have controlled data on which to base therapeutic decisions.”
Sixteen years later, we have that outbreak. Thousands are dead, millions are panicking, our economy is in shambles, and people are drinking aquarium cleaning solution in desperation, and we still don't have a credible treatment. The poor quality of the French trial shows the results of running a trial under frantic emergency conditions. We need a streamlined system for starting high-quality clinical trials the first week the next one hits. Waiting a year or longer for a vaccine is not an option. We especially need a better treatment for cytokine storm[6], which is a major cause of death in SARS-type diseases as well as sepsis and toxic shock. Tocilizumab may be a promising treatment, but some serious side effects have been reported, including acute hypertriglyceridemia and pancreatitis.
What makes trials so difficult is the short-lived and life-threatening nature of the disease. A better way of running clinical trials is needed that takes this into account.
1. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B,
Courjon J, Giordanengo V, Vieira VE, Dupont HT, Honoré S, Colson P,
Chabrière E, La Scola B, Rolain JM, Brouqui P, Raoult D (2020).
Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an
open-label non-randomized clinical trial.
Int J Antimicrob Agents. Mar 20, 105949.
doi: 10.1016/j.ijantimicag.2020.105949. PMID: 32205204
Note: this article
is
undergoing additional post-publication peer review.
2. Gao J, Tian Z, Yang X (2020). Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. Mar 16; 14(1):72–73. doi: 10.5582/bst.2020.01047. PMID: 32074550 Link
3. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C (2020).A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. Mar 18. doi: 10.1056/NEJMoa2001282. PMID: 32187464
4. Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, Seidah NG, Nichol ST. (2005). Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2, 69. PMID: 16115318 PMCID: PMC1232869 DOI: 10.1186/1743-422X-2-69
5. Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M. (2004). In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 323(1), 264–268. PMID: 15351731 DOI: 10.1016/j.bbrc.2004.08.085
6. Muller MP, McGeer A, Straus SE, Hawryluck L, Gold WL. (2004). Clinical trials and novel pathogens: lessons learned from SARS. Emerg Infect Dis. 10, 389–394. PMID: 15109402
7. Gerlach H (2016). Agents to reduce cytokine storm F1000Res. 5,2909. doi: 10.12688/f1000research.9092.1 PMCID: PMC5224679 PMID: 28105327
8. Nigrovic PA, Schneider R, (2019). Systemic juvenile idiopathic arthritis and adult onset Still disease in Hashkes PJ, Laxer RM, Simon A, eds. (2019) Textbook of autoinflammation, Springer, Switzerland, p 604.
9. Lachmann HJ (2019). Corticosteroid, other biologic and small molecule therapies in systemic autoinflammatory disorders. in Hashkes PJ, Laxer RM, Simon A, eds. (2019) Textbook of autoinflammation, Springer, Switzerland, p 604.
10. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Sevestre J, Mailhe M, Doudier B, Aubry C, Amrane S, Seng P, Hocquart M, Finance J, Vieira Ve, Dupont HT, Honoré S, Stein A, Million M, Colson P, La Scola B, Veit V, Jacquier A, Deharo JC, Drancourt M, Fournier PE, Rolain JM, Brouqui P, Raoult D (2020). Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: an observational study https://www.mediterranee-infection.com/wp-content/uploads/2020/03/COVID-IHU-2-1.pdf. Downloaded 03/27/2020
11. 陈军, 刘丹萍, 刘莉, 刘萍,
徐庆年, 夏露, 凌云, 黄丹,
宋树丽, 张丹丹, 钱志平,
李涛, 沈银忠, 卢洪洲 (2020).
硫酸羟氯喹治疗普通型
2019 冠状病毒病(COVID-19)患者
初步研究
Chen J, Liu D, Liu L, Liu P, Xu Q, Xia L, Ling Y, Huang D, Song S, Zhang D,
Qian Z, Li T, Shen Y, Lu H (2020). A pilot study of hydroxychloroquine in treatment
of patients with common coronavirus disease-19 (COVID-19)
Journal of Zhejiang University
http://subject.med.wanfangdata.com.cn/UpLoad/Files/202003/43f8625d4dc74e42bbcf24795de1c77c.pdf
Link
doi: 10.3785/j.issn.1008-9292.2020.03.03 [Chinese w/ English abstract]
12. Deng L, Li C, Zeng Q, Liu X, Li X, Zhang H, Hong Z, Xia J (2020). Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study. J Infect. Mar 11. pii: S0163-4453(20)30113-4. doi: 10.1016/j.jinf.2020.03.002. PMID: 32171872
13. Machado MO, Oriolo G, Bortolato B, Köhler CA, Maes M, Solmi M, Grande I, Martín-Santos R, Vieta E, Carvalho AF (2017). Biological mechanisms of depression following treatment with interferon for chronic hepatitis C: A critical systematic review. J Affect Disord. 209, 235–245. doi: 10.1016/j.jad.2016.11.039. PMID: 27936453
14. Dominguez-Villar M, Baecher-Allan CM, Hafler DA. (2011). Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 17, 673–675. doi: 10.1038/nm.2389. PMID: 21540856
15. Chen Z, Hu J, Zhang Z, Jiang S, Han S, Yan S, Zhuang R, Hu B, Zhang Z (2020). Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv Link https://www.medrxiv.org/content/10.1101/2020.03.22.20040758v2
16. Molina JM, Delaugerre C, Goff JL, Mela-Lima B, Ponscarme D, Goldwirt L, de Castro N (2020)., No Evidence of Rapid Antiviral Clearance or Clinical Benefit with the Combination of Hydroxychloroquine and Azithromycin in Patients with Severe COVID-19 Infection. Medecine et Maladies Infectieuses, doi: https://doi.org/10.1016/j.medmal.2020.03.006 preprint
17. J. Grein, N. Ohmagari, D. Shin, G. Diaz, E. Asperges, A. Castagna, T. Feldt, G. Green, M.L. Green, F.-X. Lescure, E. Nicastri, R. Oda, K. Yo, E. Quiros-Roldan, A. Studemeister, J. Redinski, S. Ahmed, J. Bernett, D. Chelliah, D. Chen, S. Chihara, S. Cohen, J. Cunningham, A. Monforte, S. Ismail, H. Kato, G. Lapadula, E. L'Her, T. Maeno, S. Majumder, M. Massari, M. Mora-Rillo, Y. Mutoh, D. Nguyen, E. Verweij, A. Zoufaly, A.O. Osinusi, A. DeZure, Y. Zhao, L. Zhong, A. Chokkalingam, E. Elboudwarej, L. Telep, L. Timbs, I. Henne, S. Sellers, H. Cao, S.K. Tan, L. Winterbourne, P. Desai, R. Mera, A. Gaggar, R.P. Myers, D.M. Brainard, R. Childs, and T. Flanigan (2020). https://www.nejm.org/doi/full/10.1056/NEJMoa2007016 Compassionate Use of Remdesivir for Patients with Severe Covid-19 New Engl. J. Med, April 10, 2020 DOI: 10.1056/NEJMoa2007016
18. Qiong Zhou, Xiao-Shan Wei, Xuan Xiang, Xu Wang, Zi-Hao Wang, Virginia Chen, Casey P Shannon, Scott J Tebbutt, Tobias R Kollmann, Eleanor N Fish (2020). Interferon-a2b treatment for COVID-19 https://www.medrxiv.org/content/10.1101/2020.04.06.20042580v1 doi: https://doi.org/10.1101/2020.04.06.20042580
19. Mahévas, M., Tran, V.-T., Roumier, M., Chabrol, A., Paule, R., Guillaud, C., Gallien, S., Lepeule, R., Szwebel, T.-A., Lescure, X., Schlemmer, F., Matignon, M., Khellaf, M., Crickx, E., Terrier, B., Morbieu, C., Legendre, P., Dang, J., Schoindre, Y., Pawlotski, J.-M., Michel, M., Perrodeau, E., Carlier, N., Roche, N., De Lastours, V., Mouthon, L., Audureau, E., Ravaud, P., Godeau, B., Costedoat, N. (2020). No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID-19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial https://www.medrxiv.org/content/10.1101/2020.04.10.20060699v1.full.pdf 10.1101/2020.04.10.20060699
20. Zhang, L., Sun, Y., Zeng, H.-L., Peng, Y., Jiang, X., Shang, W.-J., Wu, Y., Li, S., Zhang, Y.-L., Yang, L., Chen, H., Jin, R., Liu, W., Li, H., Peng, K., Xiao, G. Calcium channel blocker amlodipine besylate is associated with reduced case fatality rate of COVID-19 patients with hypertension DOI 10.1101/2020.04.08.20047134 https://www.medrxiv.org/content/10.1101/2020.04.08.20047134v1.full.pdf
21. Tang, W., Cao, Z., Han, M., Wang, Z., Chen, J., Sun, W., Wu, Y., Xiao, W., Liu, S., Chen, E., Chen, W., Wang, X., Yang, J., Lin, J., Zhao, Q., Yan, Y., Xie, Z., Li, D., Yang, Y., Liu, L., Qu, J., Ning, G., Shi, G., Xie, Q. (2020) Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial DOI 10.1101/2020.04.10.20060558 https://www.medrxiv.org/content/10.1101/2020.04.10.20060558v1.full.pdf
22. Liu, Q., Fang, X., Tian, L., Chen, X., Chung, U., Wang, K., Li, D., Dai, X., Zhu, Q., Xu, F., Shen, L., Wang, B., Yao, L., Peng, P. (2020). The effect of Arbidol Hydrochloride on reducing mortality of Covid-19 patients: a retrospective study of real world date from three hospitals in Wuhan https://www.medrxiv.org/content/10.1101/2020.04.11.20056523v1.full.pdf doi 10.1101/2020.04.11.20056523
23. Meng, Z., Wang, T., Li, C., Chen, X., Li, L., Qin, X., Li, H., Luo, J. An experimental trial of recombinant human interferon alpha nasal drops to prevent coronavirus disease 2019 in medical staff in an epidemic area https://www.medrxiv.org/content/10.1101/2020.04.11.20061473v1 doi 10.1101/2020.04.11.20061473
24. Magagnoli, J., Narendran, S., Pereira, F., Cummings, T., Hardin, J. W., Sutton, S. S., Ambati, J. (2020). Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19 doi 10.1101/2020.04.16.20065920 https://www.medrxiv.org/content/10.1101/2020.04.16.20065920v1
25. Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M, Mourão MPG, Brito-Sousa JD, Baía-da-Silva D, Guerra MVF, Hajjar LA, Pinto RC, Balieiro AAS, Pacheco AGF, Santos JDO Jr, Naveca FG, Xavier MS, Siqueira AM, Schwarzbold A, Croda J, Nogueira ML, Romero GAS, Bassat Q, Fontes CJ, Albuquerque BC, Daniel-Ribeiro CT, Monteiro WM, Lacerda MVG (2020). Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection A Randomized Clinical Trial. JAMA Network Open. 3(4.23):e208857. doi:10.1001/jamanetworkopen.2020.8857
26. Smit C, Peeters MYM, van den Anker JN, Knibbe CA (2020). Chloroquine for SARS-CoV-2: Implications of Its Unique Pharmacokinetic and Safety Properties. Clin Pharmacokinet. Apr 18. doi: 10.1007/s40262-020-00891-1.
27. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin J, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan, S, Yang C, Mei C, Wang, Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden F, Horby P, Cao B, Wang C (2020). Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31022-9/fulltext DOI:https://doi.org/10.1016/S0140-6736(20)31022-9
28. Yu, B., Wang, D. W., Li, C. (2020). Hydroxychloroquine application is associated with a decreased mortality in critically ill patients with COVID-19 https://www.medrxiv.org/content/10.1101/2020.04.27.20073379v1.full.pdf 10.1101/2020.04.27.20073379
29. Huang M, Li M, Xiao F, Liang J, Pang P, Tang T, Liu S, Chen B, Shu J, You Y, Li Y, Tang M, Zhou J, Jiang G, Xiang J, Hong W, He S, Wang Z, Feng J, Lin C, Ye Y, Wu Z, Li Y, Zhong B, Sun R, Hong Z, Liu J, Chen H, Wang X, Li Z, Pei D, Tian L, Xia J, Jiang S, Zhong N, Shan H (2020) Preliminary evidence from a multicenter prospective observational study of the safety and efficacy of chloroquine for the treatment of COVID-19. MedrXiv preprint. doi: https://doi.org/10.1101/2020.04.26.20081059. Not yet certified by peer review
30. Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, Labella A, Manson D, Kubin C, Barr RG, Sobieszczyk ME, Schluger NW. (2020). Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. May 7. doi: 10.1056/NEJMoa2012410. PMID: 32379955 https://www.nejm.org/doi/10.1056/NEJMoa2012410
mar 26 2020, 12:27 pm. last updated may 08 2020, 8:12 am
