 y now, most people have heard of furin, the protease whose cleavage site
mysteriously turned up in the spike protein of SARS-CoV-2. Furin is also
the culprit in many pathogens and diseases, including dengue fever, HIV,
avian influenza, papillomavirus [1], RSV (respiratory syncytial
virus), ebola, dementia, anthrax, Shiga toxin, and diphtheria toxin.
y now, most people have heard of furin, the protease whose cleavage site
mysteriously turned up in the spike protein of SARS-CoV-2. Furin is also
the culprit in many pathogens and diseases, including dengue fever, HIV,
avian influenza, papillomavirus [1], RSV (respiratory syncytial
virus), ebola, dementia, anthrax, Shiga toxin, and diphtheria toxin.
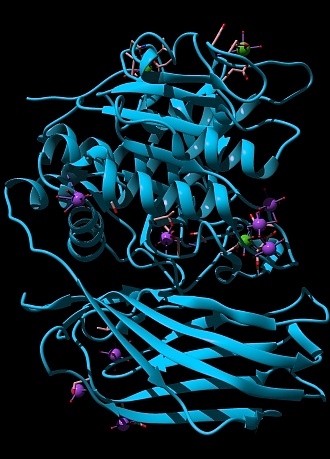
3D structure of furin after its double self-bris [2], rendered in Chimera [3], showing its vaguely Pac-Man like structure
Furin is also involved in many types of cancer including head and neck squamous cell carcinoma, some types of breast cancer, and rhabdomyosarcoma.[4] It's thought to promote the malignant phenotype of cancer cells, leading to suggestions of blocking furin as a way of treating cancer.[5]
Furin and Covid
Furin cleaves the spike protein in SARS-CoV-2, releasing the S2 subunit, which is essential for the virus to enter the cell. TMPRSS2, another protease enzyme, cleaves the same site, so any pharmacological treatment would have to block both of these enzymes at the same time.
The lethality of H5N1 influenza, which kills two-thirds of infected patients, is partly due to the fact that H5N1 has a tandem furin cleavage site. But why do we have furin, and what does it do normally when it's not trying to kill us?
Not-too-pathogenic things done by furin
Furin is not just there as a way for nature to kill us. Many proteins are made in an inactive state called a pro-protein and need to be activated by proteolysis. This is furin's role when it's not too busy letting deadly viruses and toxins into our cells.
Like an over-enthusiastic rabbi, furin performs a sort of “bris” on over 100 different proteins, removing a small but important piece of the protein, allowing it to become active. Furin even proteolyzes itself, a process called autoactivation. Performing a bris on oneself might sound like a reckless act of bravado, but furin is such a daring enzyme it does it twice, a tactic often called “measure once, cut twice.” The process of clipping off segments of pro-proteins to make them become active is called maturation.
For example, furin cleaves neurotrophins like β-NGF (nerve growth factor) and BDNF (brain-derived neurotrophic factor), turning them into their mature, active form. Furin is also essential for mature IGF-1 (insulin-like growth factor) and insulin receptor. Indeed, Fernandez et al.[6] speculated that increased furin in the blood could be the body's attempt to create more insulin receptors to compensate for insulin resistance in diabetes.
Furin requires calcium for its activity. Potassium makes furin more active, so we might expect furin to be found only inside the cell, but no: it cleaves some proteins at the cell surface, like anthrax toxin PA (protective antigen) and SARS-CoV-2 spike, but cleaves others, like influenza virus hemagglutinin, in the trans-Golgi network (TGN) inside the cell. Wherever you go, there is furin chewing up proteins.
Furin in the brain
Furin also cleaves Notch, a protein that is essential for at least 148 different things, and it cleaves both α- and β-secretases, which control whether β-amyloid is produced in Alzheimer's disease. Furin is involved in development and has its tentacles deep in the immune system.
A colleague of mine even discovered that ApoE4, the protein that puts people at high risk for Alzheimer's, prevents furin from activating BDNF, thereby interfering with the brain's self-repair mechanism. Low BDNF levels are associated with depression and loss of synapses, both of which occur in Alzheimer's disease. Without furin, we'd all be depressed and we'd probably also have dementia.
(Incidentally, my university got rid of that guy in a fit of pique against his former boss. Because of the moronic behavior of these academic bureaucrats, our country lost this brilliant scientist forever: he returned to his home country and is now the director of an institute there—and working on Covid.)
Hypoxia and hypoxemia
Hypoxemia, or low blood oxygen, is a classic finding in Covid-19, and it's a potent inducer of furin. It works through a protein called hypoxia-inducible factor-1 or HIF-1, which does many things including binding to your DNA and activating the FUR gene, resulting in expression of more furin.[9] So it's possible that, by causing hypoxia, Covid causes the synthesis of more furin, leading to a worse infection and even more hypoxia.[6] So far as I know, no one has tested this yet.
Furin levels are also elevated in diabetes, hyperlipidemia, hypertension, and obesity, and it's been suggested that this is why these factors increase mortality for Covid.[7]
Can we get rid of furin?
Many furin inhibitors have been invented, most notably peptide-based compounds
like alpha1-PDX and decanoyl-arg-val-lys-arg-CH2Cl,
but blocking such an important enzyme could have widespread and harmful side
effects. As mentioned above, furin is essential for BDNF and NGF maturation,
so if the inhibitor got into the nervous system it would be quite harmful.
Heparin inhibits furin,[8] and some people have suggested giving
heparin to Covid patients for this reason, as Covid also increases blood
coagulation (doctors tell me they've all become sudden experts on D-dimers)
so heparin would be killing two birds at once.
Flavonoids such as luteolin, baicalein, chrysin, and oroxylin are also said to inhibit furin.[7] Because, as mentioned above, hypoxia is such a potent inducer of furin, it's been suggested that HIF-1 might be a better drug target. But interfering with HIF-1 could be just as risky: it might interfere with the body's response to hypoxia. If furin does everything, as it appears to do, then interfering with furin could interfere with anything. It seems we can't live with furin and we can't live without it.
1. Day PM, Schiller JT (2009). The role of furin in papillomavirus infection. Future Microbiol. (10),1255–1262. doi: 10.2217/fmb.09.86. PMID: 19995186; PMCID: PMC2829937.
2. Dahms SO, Arciniega M, Steinmetzer T, Huber R, Than ME (2016). Structure of the unliganded form of the proprotein convertase furin. Proc Natl Acad Sci U S A 113: 11196–11201
3. UCSF Chimera was developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from NIH P41-GM103311. Citation: UCSF Chimera--a visualization system for exploratory research and analysis. Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004). J Comput Chem. 25(13), 1605–1612. https://www.cgl.ucsf.edu/chimera/
4. Jaaks P, Bernasconi M (2017). The proprotein convertase furin in tumour progression. Int J Cancer. 141(4), 654–663. doi: 10.1002/ijc.30714. PMID: 28369813.
5. He Z, Khatib AM, Creemers JWM (2020). Loss of the proprotein convertase Furin in T cells represses mammary tumorigenesis in oncogene-driven triple negative breast cancer. Cancer Lett. 484:40–49. doi: 10.1016/j.canlet.2020.05.001. PMID: 32389711.
6. Fernandez C, Rysä J, Almgren P, Almgren P, Nilsson J, Engström G, Orho-Melander M, Ruskoaho H, Melander O (2018). Plasma levels of the proprotein convertase furin and incidence of diabetes and mortality. J Intern Med 284(4), 377–387. DOI: https://doi.org/10.1111/joim.12783
7. Fitzgerald K (2020). Furin Protease: From SARS CoV-2 to Anthrax, Diabetes, and Hypertension. Perm J. 24, 20.187. doi: 10.7812/TPP/20.187. PMID: 33202215; PMCID: PMC7671676.
8. Belen-Apak FB, Sarialioglu F (2020). The old but new: Can unfractioned heparin and low molecular weight heparins inhibit proteolytic activation and cellular internalization of SARS-CoV2 by inhibition of host cell proteases? Med Hypotheses 142:109743. DOI: https://doi.org/10.1016/j.mehy.2020.109743.
9. McMahon S, Grondin F, McDonald PP, Richard DE, Dubois CM (2005). Hypoxia-enhanced expression of the proprotein convertase furin is mediated by hypoxia-inducible factor-1: impact on the bioactivation of proproteins. J Biol Chem. 280(8), 6561–6569. doi: 10.1074/jbc.M413248200. PMID: 15611046.
jan 17 2021, 4:21 am
