 T-PCR is often called the “gold standard” for testing for the
presence of SARS-CoV-2 virus. But many people are claiming that up to 97% of
PCR tests are false positives or false negatives. What is the truth?
There are several points to keep in mind.
T-PCR is often called the “gold standard” for testing for the
presence of SARS-CoV-2 virus. But many people are claiming that up to 97% of
PCR tests are false positives or false negatives. What is the truth?
There are several points to keep in mind.
First, it is practically tautological that detection of a virus by PCR does not equate to an infection. Physicians generally agree that for a person to have a COVID infection, signs of an infection must actually be present. If a patient has symptoms of a lung infection (coughing, positive CT scan, etc.), then it makes sense to give them a PCR test to determine whether the infection is COVID, influenza, or something else. And yes, there are things much worse than COVID and influenza.
Second, a detection of viral RNA on a patient, even if it was obtained correctly, is not as informative as detection of virus in the patient's blood. This rather vague statement of the obvious needs to be put into numbers, and the legendary virologist Didier Raoult and his colleagues have provided us with those numbers in a 2020 article in Clinical Infectious Diseases[1].
Below is their graph showing the percentage of patients who ultimately became positive for COVID-19 vs Ct, or cycle threshold, which is the number of PCR cycles needed to see a specified signal, as described here.
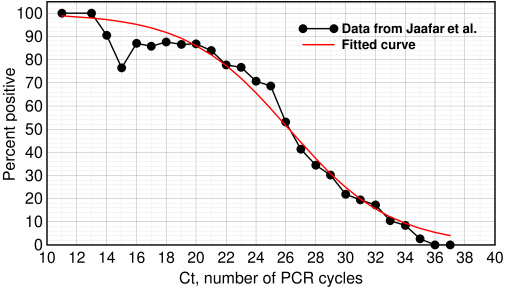
Percent infected vs Ct value. Redrawn from Jaafar et al.[1] with a logistic curve (red curve) fitted to the data
These doctors aren't mathematicians: they incorrectly drew a polynomial curve instead of a logistic curve through their data, but their experimental results, based on over a quarter million COVID RT-PCR tests in their institute, are still informative. Of the 3,791 patients who tested positive by PCR, they were able to isolate SARS-CoV-2 on 1,941. They reported that at Ct=25, about 70% of patients remained positive in culture. At Ct=30 and 35, only 20% and 3% respectively were positive.
This is essential information. What does this mean? It does not mean that 97% of the patients were never infected or that they will never get the disease. It only says that evidence of infection could not be found even though the patients tested ‘positive.’ The virologists state that further experiments will be needed to determine what is happening.
The problem is with the word ‘positive.’ A PCR test is probabilistic. A false positive could result from detecting inactive or fragmented viruses or it could mean the patient's immune system successfully eliminated it. There have even been a few cases where a patient tested negative or with a Ct >35 and yet eventually got the disease.
A PCR result without a Ct score is almost meaningless. Ct values should be provided routinely to patients, along with a copy of the logistic curve, so they can make an informed decision.
A report calls for retraction of the RT-PCR paper
The COVID RT-PCR test is described here[2].

Elevator sign limiting occupancy to two. There are no signs yet telling us how many are allowed in the stairways. Update A new sign just went up: 2.
So, what to make of a new report[3] that appeared a few days ago questioning its credibility? Essentially this report is a 75-page post-publication peer review. The authors claim that the test is flawed because the original authors did not validate the size of the PCR product, the primer concentrations were way too high, they did not use positive and negative controls, they used too many cycles, their primers were badly designed, and they did not provide an SOP to ensure that other labs used the same procedure. In short, everything that was possible to do wrong they did wrong.
Using excessively high primer concentrations or exceeding 25 cycles can lead to non-specific amplification and would cause false positives. If it is true that the authors did not check the size of the product, it is a damning indictment, as there would be no assurance that they were amplifying the right product.
The authors say this test is the basis of all current COVID-19 testing. They say, correctly, that a positive/negative result is insufficient. Labs must provide the Ct value; without it, RT-PCR is unsuitable as a specific diagnostic tool.
Conclusion
It is a widely held perception that academic labs tend to be “cowboys”: they don't always do the tedious validation steps that clinical and industry labs are required to do. I have often been surprised to see grad students and others grab an assay from the literature, publish a result, and move on to something else, not realizing they are contributing to the so-called reproducibility problem in science.
But academics are not to blame for an unreliable test: you cannot survive in academia if you try to use GLP. The reliability of a clinical test depends not on some academic paper, but on rigorous validation by manufacturers and the CDC and strict adherence to protocols in clinical labs. Nonetheless, people should recognize that even a properly-conducted PCR test is not determinative: it only gives a rough probability that a virus may be present. From a biochemical perspective, RT-PCR barely even qualifies as a quantitative assay, but as a screening tool it is better than many of the proposed alternatives.
RT-PCR is what it is. What is happening is that administrators and politicians are hiding behind the PCR test, giving it more credibility than it deserves. Their goal is to avoid being blamed if someone in their area gets the disease. If they get criticized, they blame the test. This is a problem for the voters, not clinical labs.
1. Jaafar R, Aherfi S, Wurtz N, Grimaldier C, Hoang VT, Colson P, Raoult D, La Scola B. Correlation between 3790 qPCR positives samples and positive cell cultures including 1941 SARS-CoV-2 isolates. Clin Infect Dis. 2020 Sep 28:ciaa1491. doi: 10.1093/cid/ciaa1491. PMID: 32986798; PMCID: PMC7543373. full-text link
2. Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T, Brünink S, Schneider J, Schmidt ML, Mulders DG, Haagmans BL, van der Veer B, van den Brink S, Wijsman L, Goderski G, Romette JL, Ellis J, Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MP, Drosten C. (2020). Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020 Jan;25(3):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. Erratum in: Euro Surveill. 2020 Apr;25(14): Erratum in: Euro Surveill. 2020 Jul;25(30): PMID: 31992387; PMCID: PMC6988269.
3. https://cormandrostenreview.com/report/ Review report Corman-Drosten et al. Eurosurveillance 2020 November 27, 2020
dec 02 2020, 6:33 am
