Introduction
Can You Survive on Soap?
 by T Nelson
by T Nelson
 uppose you were on a desert island and all you had to eat was soap.
What would happen? Could it keep you alive?
uppose you were on a desert island and all you had to eat was soap.
What would happen? Could it keep you alive?
It's one of those things that might be handy to know. But surprisingly, there has been very little research on this subject. Soap contains a mixture of fatty acids that have been produced by reacting fats with a strong base—usually sodium hydroxide—in a process called saponification. A fatty acid is a long hydrocarbon chain with a carboxylic acid (CO2H) group at one end. Stearic acid (see figure) is a typical example. Most of the time, these fatty acids are attached to glycerol by ester bonds, forming triglycerides, which are the predominant form in which fatty acids are stored in the body. Strong base breaks these ester bonds and in so doing converts the fatty acid to a salt, in which the negative charge of the carboxylic acid is neutralized with a sodium ion. Liquid soaps often contain potassium instead of sodium. While the product is not particularly nutritious, it does contain energy that, in principle, could be used by the body to stay alive.
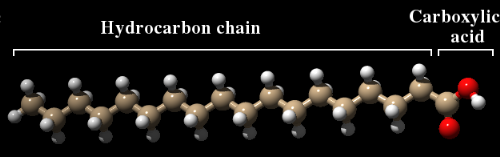
Stearic acid. Brown=carbon, Red=oxygen, White=hydrogen. The carboxylic acid has a negative charge, while the hydrocarbon chain is neutral.
Pure soap is not poisonous, but its physico-chemical properties would cause major digestive problems. Because it's basic, it would neutralize your stomach acid. It could even damage the membranes in the cells in the small intestine. This happens because the cell membranes are made of a combination of cholesterol and fatty acids bound to a glycerol backbone: triglycerides again. Soap or detergent disrupts this structure, and even though small intestinal cells are fairly rugged as cells go, they can still be damaged. If that happens, the fatty acids would not be absorbed, and other bad things could happen.
In earlier times, soaps were made from animal fat. This kind of soap contained essential fatty acids, such as linoleic and linolenic acid, which are essential for life. However, nowadays manufacturers destroy these fatty acids by hydrogenation, which simply means adding two hydrogen atoms to each carbon-carbon double bond. This prevents the soap from reacting with oxygen, which would cause the fat to turn rancid.
Incidentally, this reaction with oxygen is one reason linseed oil is no longer used. The reaction generates heat. In the past, rags soaked with linseed oil would get so hot from spontaneous reaction with oxygen that they started a fire.
Detergents are chemically more homogeneous. Instead of a carboxylic acid group, detergents contain a sulfate, sulfonate or phosphate group, which is also negatively charged. Detergents containing a sulfonate group are called linear alkylbenzenesulfonates, which means the sulfate is attached to the carbon chain via a 6-membered ring similar to toluene, given the generic name of alkylbenzene. Another type of detergent called quaternary ammonium detergents are positively charged. A third type is non-ionic detergents, which are used by laboratories and drug companies. All of these are difficult to absorb and have little or no nutritional value.
Laundry detergent also may contain large amounts of phosphate (which is being phased out), zeolites (which are indigestible), and sodium carbonate, which has no nutritional value, and up to 7% bleach, such as hypochlorite or perborate.
It is the basicity of the sodium hydroxide, not the fatty acids in the soap, that actually does most of the cleaning. The fatty acid chains contribute to the cleaning action by dissolving some materials that are insoluble in water.
This basicity is what makes soaps hard for the intestine to absorb. However, soap can be neutralized by adding a weak acid such as vinegar or lemon juice. This converts the soap to a foamy mess. It may be unappetizing, but if you're in a survival situation, it might save your life. Of course, it's important to get the proportion of soap and acid right. Drinking something acidic, or even biting into a lemon, can destroy the enamel on your teeth within seconds.
Some soaps and detergents contain extra ingredients that could be toxic. In one case, a patient in India got barium poisoning from eating a soap product that contained barium. In the USA, many soaps contain titanium dioxide, a white pigment that is biologically inert.
Soap will not keep you alive for long. It contains no protein or other nutrients. However, some laundry detergents contain up to 2% enzymes, which makes them a very small, albeit not very tasty, source of protein—if you can figure out how to separate the proteins from the detergents, which can kill you. It might not be tasty, but it if you're starving, it beats the heck out of shoe leather.
You probably never realized that when your parents were making you wash out your mouth with soap, they were really giving you elementary survival training.